实验案例
产品推荐
新闻推荐
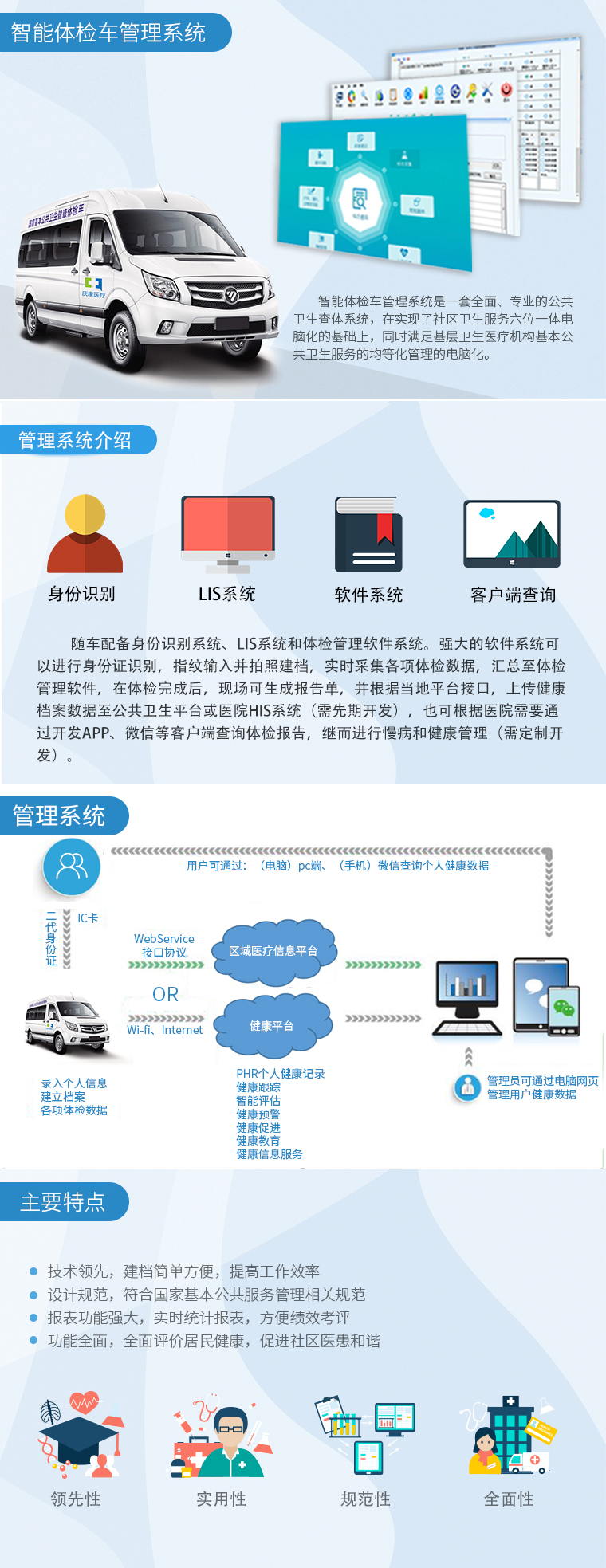
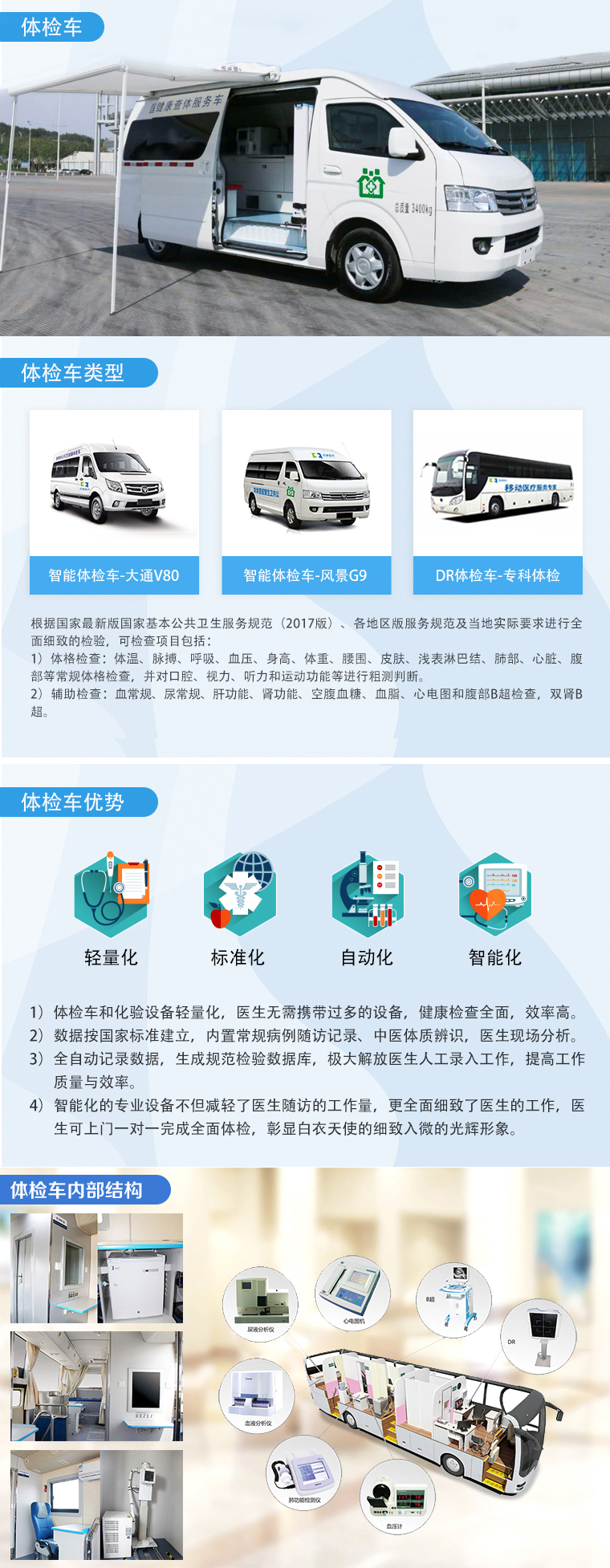
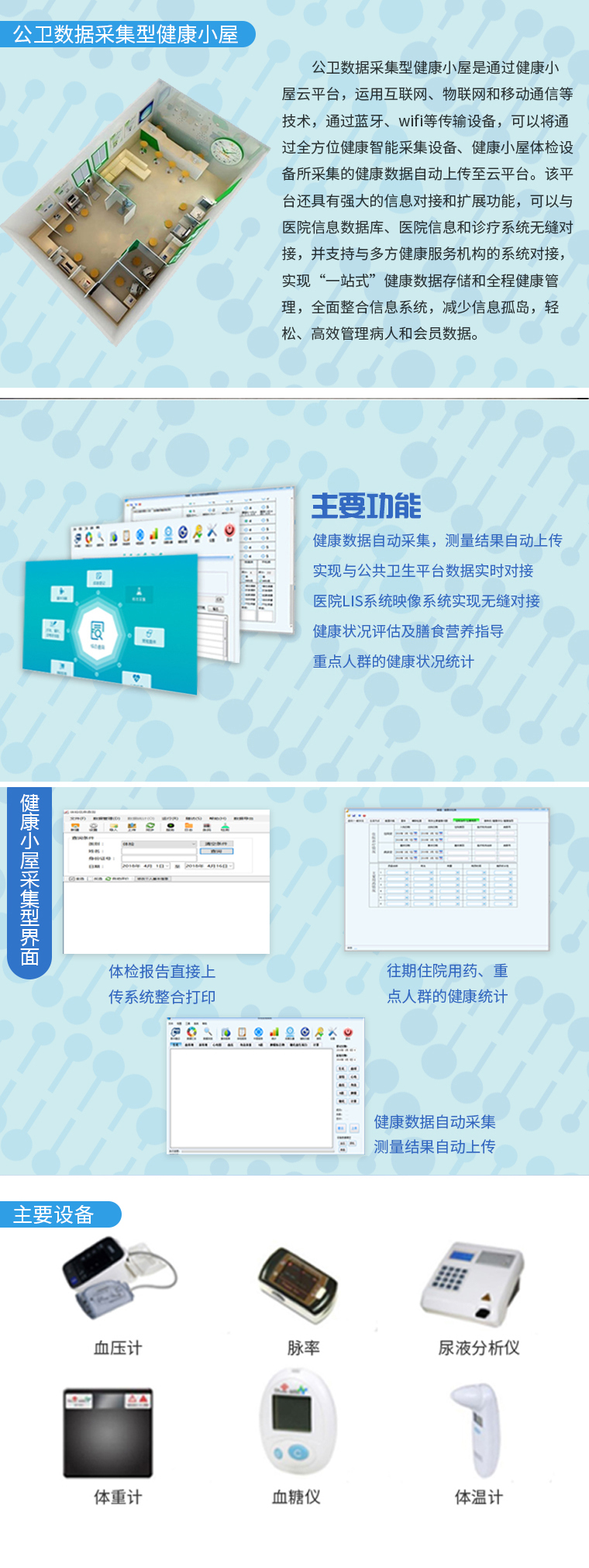
疾病预防控制中心检测治理信息系统
- 2023-10-16
- / 原创
- 130
In recent years, in the field of disease prevention and control, public health incidents have been widely and deeply explored through the application of information technology in daily work. Accelerating the process of information construction in disease control laboratories can effectively control each node in different types of testing tasks, prevent multiple information errors in different systems, hinder approval and decision-making efficiency, and accumulate effective data resources, The Shengyuan Guangtong Disease Prevention and Control Center Testing and Management Information System, as a means of preventing major risks in the field of health from the source, provides technical specifications for information sharing in health monitoring, disease prevention and control testing, emergency response, and quality control.
疾控中心实验室面临的相关问题有:
The relevant issues faced by the CDC laboratory include:
一、治理衔接不细密,,,
,,,导致在整个实验室治理历程中执行职员保存误差,,,
,,,导致事情效率低;
;
;;;
1、 The lack of close management connection leads to deviations in the execution of personnel throughout the entire laboratory management process, resulting in low work efficiency;
二、样本的治理流程不完善,,,
,,,吸收、流转、贮存、处置惩罚等溯源难题;
;
;;;
2、 The management process of samples is incomplete, and it is difficult to trace the source of receipt, circulation, storage, and disposal;

三、数据溯源是科研效果的包管,,,
,,,数据衔接不实时导致信息差;
;
;;;
3、 Data traceability is the guarantee of scientific research achievements, and untimely data connection leads to poor information;
四、多部分协同进度条希望纷歧致亦或者重复性事情,,,
,,,报告出具慢;
;
;;;
4、 The progress of multi department collaboration progress bars is inconsistent or repetitive, and the report issuance is slow;
五、各实验室节点的情形差别步,,,
,,,情形清静防护监测不到位容易引发清静事故
。。。。
5、 The situation of each laboratory node is not synchronized, and inadequate environmental safety protection monitoring can easily lead to safety accidents.
产品特征:
Product characteristics:
一、无邪搭载种种仪器装备知足实验室需求
1、 Flexibly equipped with various instruments and equipment to meet laboratory needs
二、切合实验室种种规范
2、 Comply with various laboratory specifications
三、实现检测流程闭环追溯
3、 Realize closed-loop traceability of the testing process
四、包管化验剖析数据的严酷治理和控制
4、 Ensure strict management and control of laboratory analysis data
五、整合各检测流程环节集中治理
5、 Integrate various testing processes and centralize management
本文由区域两癌筛查治理系统友情贡献.更多有关的知识请点击:真诚的态度.为您提供为周全的效劳.更多有关的知识我们将会陆续向各人贡献.敬请期待.
This article is a friendly contribution from the regional two cancer screening management system. For more relevant knowledge, please click on: Sincere attitude. We will provide you with comprehensive services. We will gradually contribute more relevant knowledge to everyone. Stay tuned